Researchers create a new material to obtain green hydrogen more efficiently and could replace Helium
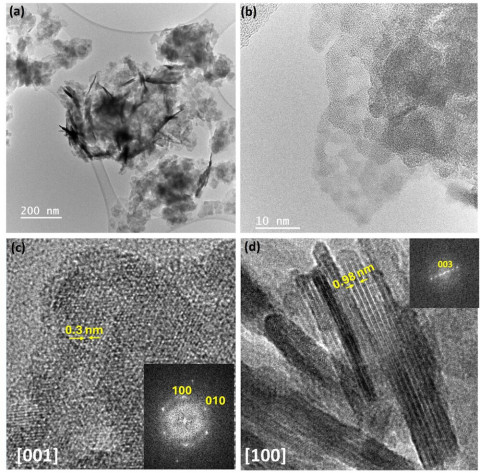
Research groups from the Madrid Institute of Materials Science ¡(ICMM-CSIC) and the Department of Inorganic Chemistry (QI) of the Faculty of Chemistry at the Complutense University of Madrid (UCM) have developed a new material with only two dimensions that has great potential for generating green hydrogen from water in a much more efficient way than current technologies. The work, which has just been published in the journal Advanced Materials, will enable better development of clean technologies.
"Hydrogen is an energy vector with a relevant role in reducing carbon emissions, but its industrial production presents major challenges," explains José Luis Martínez Peña, a CSIC researcher at ICMM and one of the lead authors of the research. Starting from this point, the working groups have created a new material that, thanks to the combination of cobalt and molybdenum oxides in just two dimensions, allows for the improvement of the kinetics of electrocatalytic processes involved in obtaining green hydrogen in a much more efficient way than current catalysts based on noble metals.
The incorporation of molybdenum into cobalt hydroxide "significantly improves" the onset potential of the Oxygen Evolution Reaction (OER) when compared to the performance of ruthenium dioxide, considered one of the best current catalysts for producing hydrogen, explains José María González-Calbet, a researcher at UCM and another lead author of the work.
But that's not all. "An unexpected characteristic of this new material has been its magnetocaloric effect at liquid hydrogen temperature," continues Martínez. This is a thermodynamic phenomenon where certain materials change their temperature simply by being exposed to a magnetic field: "This material also proves to be a highly potential alternative to current cooling methods," adds UCM researcher María Luisa Ruiz González, another lead author of the study.
This second characteristic, in fact, gives this material great potential to replace helium in its cryogenic applications. Helium is a noble gas that is 'endangered' as there are only two sources to obtain it – typically through drilling oil or gas wells in few areas of the planet – while its use is increasingly widespread for cooling large devices and for manufacturing semiconductors and batteries.
One of the strengths of this work relates to the multidisciplinary approach with which it has been tackled. The new material has been explored and analyzed using unique techniques available only at major national and international infrastructures: the National Center for Electron Microscopy (CNME) at UCM, the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, and the Diamond synchrotron in the United Kingdom.
"This study opens new perspectives for the development of functional materials based on (poly)oxomolybdates intercalated in cobalt hydroxide for generating green hydrogen from water splitting and alternatively being used as a refrigerant to reach very low temperatures close to liquid Hydrogen," conclude the scientists.
Reference:
Daniel Muñoz-Gil, Celia Castillo-Blas, Dawid Krystian Feler, Isabel Gómez-Recio, Miguel Tinoco, Ana Querejeta-Fernández, Rodrigo González-Prieto, Felipe Gándara, Romualdo Santos Silva Jr, Pilar Ferrer, Carlos Prieto, Luc Lajaunie, José Luis Martinez-Peña*, María Luisa Ruiz-González*, José María González-Calbet*. 2D Co-Mo-Hydroxide-Based Multifunctional Material for the Development of H2-Based Clean Energy Technologies. Advanced Materials. DOI: https://doi.org/10.1002/adma.202512458
Instituto de Ciencia de Materiales de Madrid (ICMM)
Sor Juana Ines de la Cruz, 3
Cantoblanco, 28049
Madrid, España
Telephone: (+34) 91 334 90 00
Email: @email
Communication Office: @email

Acknowledge the Severo Ochoa Centres of Excellence program through Grant CEX2024-001445-S/ financiado por MICIU/AEI / 10.13039/501100011033

Contacto | Accesibilidad | Aviso legal | Política de Cookies | Protección de datos
