ICMM researchers eliminate microplastics from water with iron oxide nanoflowers
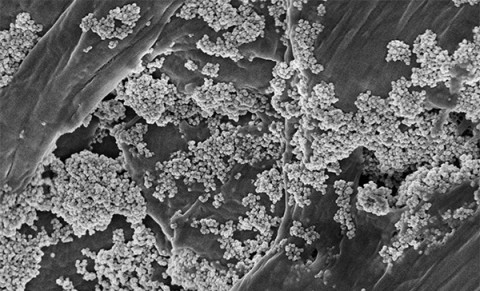
Researchers from the Materials Science Institute of Madrid (ICMM-CSIC), a center of the Spanish National Research Council (CSIC) under the Ministry of Science, Innovation, and Universities (MICIU), have developed a scalable method to produce iron oxide nanoflowers capable of extracting and degrading microplastics from cosmetics in water. The process, published in the Chemical Engineering Journal, represents a breakthrough in water decontamination techniques to make them greener and more energy-efficient.
"Currently, waste treatment plants use very large-scale and costly processes," says Álvaro Gallo-Córdova, a researcher at ICMM-CSIC and one of the principal authors of the work. This research addresses this issue by working on the nanometer scale (one-millionth of a millimeter) with processes that are "much more efficient." "Green methods produce our particles and they can also be reused," the scientist points out.
This work uses iron oxide nanoparticles in the form of nanoflowers. "The shape is important," explains Gallo-Córdova. Iron oxide is a magnetic material with a high surface area, allowing it to capture many contaminants at once. "When it is in the form of nanoflowers, it has a cooperative magnetic behavior. That is, they are particles with several cores that cooperate to enhance and improve their magnetic properties," says the researcher.
A two-stage removal process
The removal of microplastics with these nanoflowers occurs in two stages. First, they are placed on the microplastics and adhere to them "in just five minutes." "This makes the microplastics magnetic, and we remove them from the water with a magnet," notes Gallo-Córdova. "This is already a significant advancement, but our group wanted to go further: to eliminate it," he adds.
Once the microplastic is out of the water, it is hydrolyzed (a process in which the plastic particles break down into small molecules), and then, using these same nanoflowers, free radicals are produced: "These radicals are highly reactive species that degrade organic contaminants," he explains. "What you get after the process is just CO2 and water," describes the scientist, who notes that, although CO2 could currently be considered a waste, "it can be reused."
Moreover, this process occurs at low temperatures: "The nanoflowers are heated in the presence of alternating magnetic fields, and their heating is sufficient to carry out the contaminant degradation reaction without having to heat the water." This results in a double energy saving: they do not have to heat the water (currently, these processes are conducted at 90°C) nor cool it afterward to return it to nature.
"We eliminate a contaminant in a single process, faster than current processes. At an industrial level, this is quite interesting," the researcher celebrates. "These findings represent a remarkable advancement," adds Puerto Morales, also a researcher at ICMM-CSIC and an author of the study. "We have scaled up the production of these nanoparticles to gram level and reduced costs by half, so greater industrial scalability will lead to greater economic savings," she concludes.
Reference:
Instituto de Ciencia de Materiales de Madrid (ICMM)
Sor Juana Ines de la Cruz, 3
Cantoblanco, 28049
Madrid, España
Telephone: (+34) 91 334 90 00
Email: @email
Communication Office: @email

Acknowledge the Severo Ochoa Centres of Excellence program through Grant CEX2024-001445-S/ financiado por MICIU/AEI / 10.13039/501100011033

Contacto | Accesibilidad | Aviso legal | Política de Cookies | Protección de datos
