Women for quantum—manifesto of values
Almut Beige, Ana Predojević, Anja Metelmann, Anna Sanpera, Chiara Macchiavello,Christiane P. Koch, Christine Silberhorn, Costanza Toninelli, Dagmar Bruß,Elisa Ercolessi, Elisabetta Paladino, Francesca Ferlaino, Giulia Ferrini, Gloria Platero,Ivette Fuentes, Kae Nemoto, Leticia Tarruell, Maria Bondani, Marilu Chiofalo,Marisa Pons, Milena D’Angelo, Mio Murao, Nicole Fabbri, Paola Verrucchi,Pascale Senellart-Mardon, Roberta Citro, Roberta Zambrini, Rosario González-Férez,Sabrina Maniscalco, Susana Huelga, Tanja Mehlstäubler, Valentina Parigi &Verònica Ahufinger.
Communications Physics
Low-Cost WS2 Photodetector on Water-Soluble Paper for Transient Electronics
Gulsum Ersu*, Luke Doolan, Thomas Pucher, Jonathan N. Coleman, Andres Castellanos-Gomez*
Advanced Functional Materials
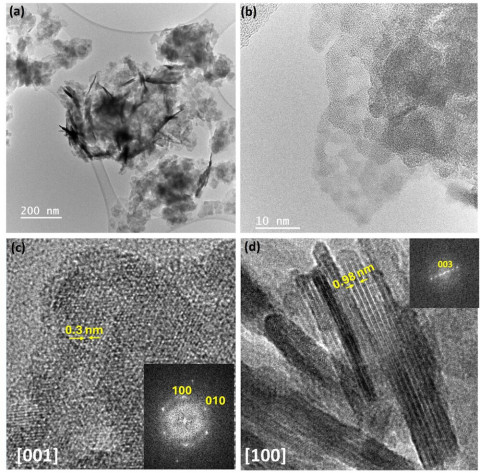
High-Yield Synthesis of Fe-NC Electrocatalysts Using Mg2+ Templating and Schiff-Base Porous Organic Polymers
Eliot Petitdemange, Jinjie Zhu, Angus Pedersen, Joseph Parker, S. Esmael Balaghi, Shaohua Li, Silvia Favero, José Ignacio Martínez, Sarah Haigh, Maria-Magdalena Titirici, Anna Fischer, Jesús Barrio
Advanced Functional Materials
News
Welcome
Instituto de Ciencia de Materiales de Madrid (ICMM)
Sor Juana Ines de la Cruz, 3
Cantoblanco, 28049
Madrid, España
Telephone: (+34) 91 334 90 00
Email: @email
Communication Office: @email

Acknowledge the Severo Ochoa Centres of Excellence program through Grant CEX2024-001445-S/ financiado por MICIU/AEI / 10.13039/501100011033

Contacto | Accesibilidad | Aviso legal | Política de Cookies | Protección de datos