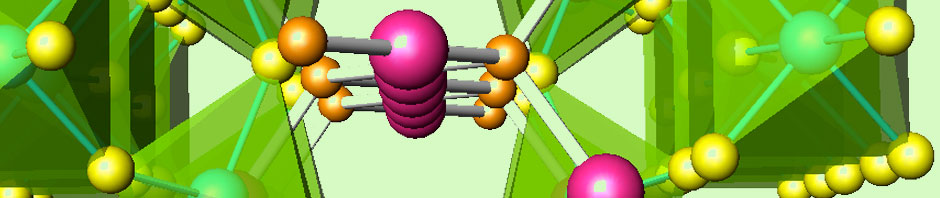
Many transition-metal oxides in unusual valence states, with exceptional electronic properties, are metastable under ambient conditions, and require special synthesis conditions such as high pressure, or the use of strongly oxidizing or reducing conditions or, in general, the utilization of moderate treatment temperatures.
Concerning their preparative chemistry, our Laboratory of the “Instituto de Ciencia de Materiales de Madrid” has been pioneering, at national level, in setting up and exploiting two different types of equipment of high pressure synthesis, which have shown to be, so far, extraordinarily fruitful.
Thus, we have accessed to certain materials which, given the difficulty of their preparation, had been little studied so far, in spite of the interesting properties they present. This gave rise to collaborations with very diverse Research Institutions, interested in the materials produced by our group.
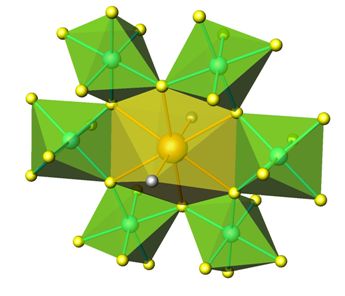
As a small overview, the high hydrostatic pressure favours the formation of the short and strongly covalent chemical bonds characterizing the high oxidation states; on the other hand, the high pressure applied by a reactive gas like oxygen provides the highly oxidizing conditions necessary for the stabilization of the mentioned valence states.
Additionally, pressure prevents the decomposition of unstable reactants at the synthesis temperature (e.g.: Tl2O3, CrO2); it helps to increase the coordination numbers and it favours the denser phases, in perovskite-like materials.
Finally, the reaction kinetics is substantially enhanced under pressure. For all these reasons, high pressure is an effective tool in the preparation of new compounds with a low stability or a metastable character.