The use of appropriately functionalized molecular building blocks to form novel nano-architectures with tailored structure and electronic properties has recently been brought under the spotlight. Recently, the ESISNA group has developed an effective way of exploiting on-surface chemistry to grow different nanostructures in sequential steps from the same molecular precursor. We have used different activation temperatures to control the on-surface C–H bond breaking and C–C bond making processes in the paradigmatic case of the nomplanar helically chiral pyridil-disubstituted dibenzo[5]helicene (1 in figure) deposited on Pt(111).
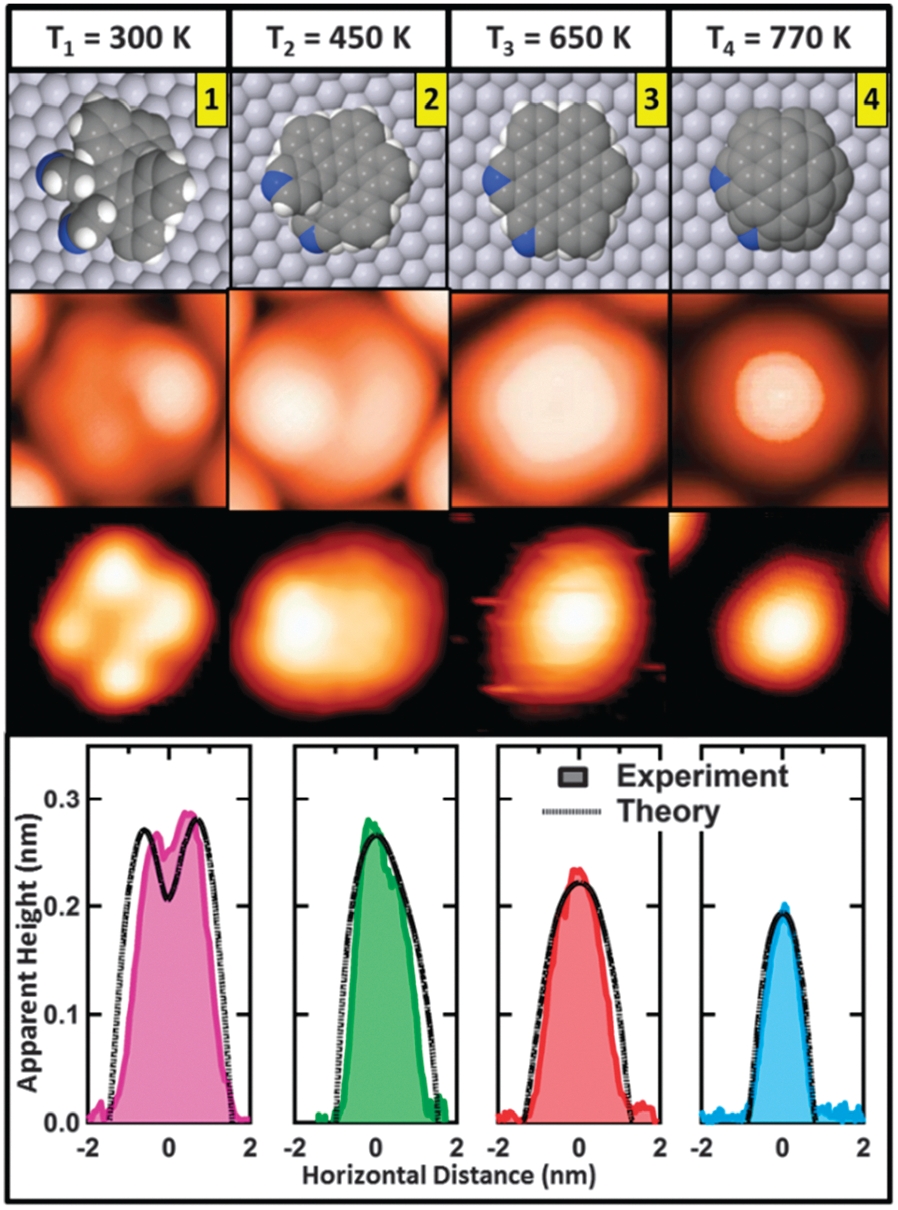
This molecule experiences dehydrogenation from different groups at different temperatures.
At 440 K it cyclodehydrogenates, by cleaving some internal C–H bonds, which subsequently close to form additional carbocycles, giving rise to N-doped nanohelicenes (2 in figure). Further annealing at 650 K cyclodehydrogenates the aza-aromatic groups to form N-d oped nanographenes (3 in figure), and finally, at 770 K rim-dehydrogenation leads to N-doped nanodomes (4 in figure) covalently bound to the metal surface. By an efficient combination of the most advanced room temperature (RT) ultra-high vacuum scanning tunneling calculations based on improved density functional theory (DFT), the sequential development microscopy (UHV-STM) technique with state-of-the-art first-principles of the different resultant structures has been monitored.
Importantly, we have found significant agreement between experimental and calculated STM images of key intermediates, which allows a detailed mapping of the individual steps of this complex on-surface transformation.
This adequate combination of experiments and theory will permit shed some light in any other thermo-induced on-surface reaction of this kind.
The results have been published in Chemical Communication 50, 1555-1557 (2014).

